- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
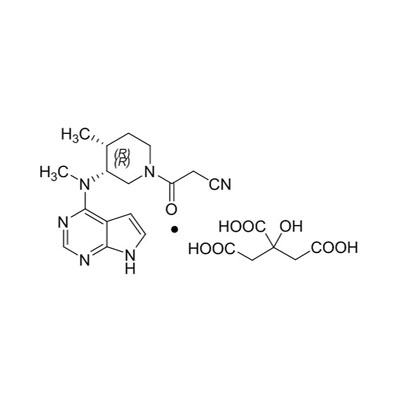
Quid facit cas no.540737-29-9 in essential media in pharmaceutical synthesis?
Cas No.540737-29-9Est summus puritas pharmaceutical intermediate late in synthesis complexu medicamento moleculis. Et ejus firmum structuram et optimum compatibility, quod ludit a crucial partes in productionem activae pharmaceutical ingredientia (Apis). Fabricari in stricto qualitas imperium signaJiangsu Run'an Pharmaceutical Co. Ltd., Hic compositis facta est momenti arbitrium pro global pharmaceutical manufacturers. Hoc articulum explorat proprietates, applications, et commoda in detail, providente clarum intellectus ad professionales quaerimus certa intermedia.
Quid Cas No.540737-29-9?
Cas no.540737-29-9 refers to a specifica eget substantia descripserunt subChemical Abstracts Service (CAS)Ratio, ensuring Traceability et Standardization. In pharmaceutical industria, ut serves ut medium compositis - a pontem inter rudis materiae et ultima apis. Ex his precise Molecular compositionem, CAS no.540737-29-9 praebet princeps stabilitatem et constantia durante eget reactiones, quae est vitalis pro assequendum reproducible results in magna-scale productio.
Hoc compositis praecipue aestimantur ad suumPuritas coerceri humorem contentus et humilis impuritas campesterFaciens idoneam petens pharmaceutical synthesis applications.
Quid est Cas No.540737-29-9 tam momenti in modern pharmaceutical vestibulum?
Pharmaceutical Synthesis saepe innititur intermedia ut utriusque efficientiam et salus in eget processibus. Cas no.540737-29-9 est discrimine intermedia quae enhances reactionem selectivity, amplio cedat, et minimizes parte profectae.
InJiangsu Run'an Pharmaceutical Co. Ltd., In compositis produci hisBene vestibulum exercitia (GMP)etISO qualitas signa, ensuring quod omne batch occurrat internationalis qualis requisita. Hoc constantia concedit pharmaceutical turmas ad confidunt in compositis in progressionem key therapeutic moleculis.
Clavis specifications et parametri
| Parameter | Specificatio |
|---|---|
| Product Name | Cas No.540737-29-9 |
| EXPLORATIO | C₁₆H₁₄n₂o₄ (Exemplum, in ipsa formula pendeat structuram) |
| Pondus | 298,29 G / Mol (approx.) |
| Pudicitia | ≥ XCIX% |
| Species | Alba ad off-album crystalline pulveris |
| Point liquescens | 120-125 ° C (Typical range) |
| Solubility | Solutum in organicis solvents (Methanol, Ethanol) |
| Conditions repono | Store in a frigus, sicco loco, arcte clausit |
| Stipare | XXV kg fibra tympanum vel customized packaging |
| Vexillum | GMM / ISO (IX) I Certified |
Quisque specificationem probatur cum provectus analytica modi utHplc, GCetNmr, ensuring plena vestigia et reproducibility.
Quid Cas No.540737-29-9 in pharmaceutical applications?
Cas No.540737-29-9 servit quodmediusIn synthesis activae pharmaceutical ingredients (Apis) et related componit. Est late in:
-
Anticancer medicamento synthesis:Ut structural intermedium conlatis ad altum bioactivity moleculis.
-
Antiviral formulae:Supporting selectivam reactiones ad formam firmum mocycular backbones.
-
Cardiovascular therapeutics:In multi-gradus synthesim efficax cordi medications.
-
Research and Development:Communiter usus est per pharmaceutical laboratories ad exploring novum medicamento moleculis.
Et compositis scriptorChemical stabilitatem et imperium reactivityFac illud maluit arbitrium in multi-gradus organicum synthesis processus.
Quid enim commoda eligendi cas no.540737-29-9 a Jiangsu Run'an Pharmaceutical Co. Ltd.?
-
Puritas et consistency:
Omne batch est produci per provectus purificationem technology, cautelas consistent perficientur. -
Strictae qualitas imperium:
Qualis probatio fit in omni productio scaena ut obsequio cum global signa. -
Flexibile Packaging et Delivery:
Lorem packaging options sunt praesto ad occursum diversis productio necessitates et translationem requisita. -
Technical Support et Documenta:
Aliquam analytica data, MSDS, et CAA sunt provisum est adiuvaret cum regulatory submissionibus et R & D verificationem. -
Firmum copia torquem:
Cum magna productio facultatem et efficiente, Jiangsu Run'an ensures opportune global partus.
Quam ut tractamus et reponunt cas no.540737-29-9 tuto?
Propriis tractantem et repono sunt discrimine ad maintaining uber integritas:
-
Vitare dirige solis et humorem nuditate.
-
Usus in bene uentilandam elit elit.
-
Copia ad locus temperatus in signatum continentia.
-
Sequantur vexillum officinarum salutem guidelines, inter caestus et goggles.
Cum tractata recte, quod productum manet chemica firmum et retinet puritatem in tempore.
FAQ: Clementine_Vulgate Quaestiones Cas No.540737-29-9
Q1: Quid prorsus est cas no.540737-29-9 usus est in pharmaceutical synthesis?
A1:Cas no.540737-29-9 praesertim usus est sicut chemical medium in productione variis pharmaceutical ingredientia. Eius princeps reactividen et structural reliability facere idoneam ad synthesim complexu moleculis ut antectancer et anticancer antecancomer.
Q2: Quid facit Jiangsu Run'an Pharmaceutical Co. Ltd. Ut Puritas Cas No.540737-29-9?
A2:In comitatu utilizes provectus analyticae instrumenta inter Hplc, GC, et Missam spectrometry ad cognoscere puritatem campester supra XCIX%. Quisque batch subeundes rigorous internum et tertius-pars temptationis ante exposch customers.
Q3: can CAS no.540737-29-9 et customized secundum elit requisita?
A3:Ita. Jiangsu Run'an Pharmaceutical Co. Ltd. offert flexibilia options pro puritate campester, packaging et labeling in occursum proprie R & D vel productio necessitates. Clients potest peto tailored documenta et sample analysis ad sanationem.
Q4: Quid sunt commendatur repono et translationem condiciones ad cas no.540737-29-9?
A4:Staed in frigus, siccis elit, protected a lumine et humorem. Per onerariis, in productum est securely signatus et tractatur secundum internationalis chemical salus signa ne contaminationem vel degradation.
Quid Elige Jiangsu Run'an Pharmaceutical Co. Ltd. ut tua confidebat socium?
Et decennia experientia in eget synthesis et pharmaceutical intermedia;Jiangsu Run'an Pharmaceutical Co. Ltd.Mobes technica peritiam cum stricto qualitas certitudinem systems. In comitatu scriptor facilities sunt instructa modern analytica laboratories et GMP-certified productio lineas, cursus reliability et innovation in omni uber.
Sive enim investigationis proposita vel industriae-scale productio, in turma providetStabilis qualitas, competitive Morbi cursus sapien et responsive auxiliumad global clients.
Conclusio
Et responsum est. Si vos es vultus parumperreliable, summus qualitas et consistent mediumNam pharmaceutical vel eget synthesis, cas no.540737-29-9 aJiangsu Run'an Pharmaceutical Co. Ltd.est optimum optionem. Puritas superior, moderatur parametri, et professional technica tergum ut ea necessaria materia in pharmaceutical agro.
Nam inquiries, quotations, aut technica notitia deCas No.540737-29-9, Quaesocontactus Jiangsu Run'an Pharmaceutical Co. Ltd.