
- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Rheumatic arthritis iguratimod
Seres Product Name: Ellamod
Seres alias: N [3 (Carboxamido) -4-Oxo-VI-phenoxy-4h-I-Benzopyran-VII-YL] Methanesulfonamide
Anglicus Product Name: Rheumatic arthritis Iguratimod
CAS # 123663-49-0
Molecular formula: c17h14n2o6s
Molecular pondus: 374,3679
Specie et proprietates alba pulveris
Domesticis Registration Number API: Y20190021542
Mitte Inquisitionem
Formula

Seres Product Name: Ellamod
Seres alias: N [3 (Carboxamido) -4-Oxo-VI-phenoxy-4h-I-Benzopyran-VII-YL] Methanesulfonamide
Anglicus Product Name: Rheumatic arthritis Iguratimod
CAS # 123663-49-0
Molecular formula: c17h14n2o6s
Molecular pondus: 374,3679
Specie et proprietates alba pulveris
Domesticis Registration Number API: Y20190021542
Rheumatic arthritis Iguratimod est pro anti-rheumatic medicamento, Synovitis medicamento.
Rheumatic arthritis Iguratimod: Applications, beneficia, et challenges
Introductio
Rheumatic arthritis Iguratimod, a Romanorum morbus-modifying antirheumatic medicamento (Dmard), quae emersit ut promittens therapeutica optionem ad administrandi Rheumatoid arthritis (Rhe). Per targeting inflammatory meatus et modulating immune respondeo, Rheumatica arthritis Iguratimod praebet unicum mechanism distincta a conventional therapies. Hoc articulum explorat orci applications, commoda, et limitations, underscoring eius crescente partes in Rheumatology.
Applications de Rheumaticae arthritis iguratimod
Primo-linea Monotherapy
Rheumatic arthritis Iguratimod est probatus est standalone treatment quia mitis est moderari ra. Volume iudiciis demonstrare ad efficaciam in reducendo iuncturam tumor, dolorem, et mane rigor a suppresso pro-inflammatione cytokines ut tnf-α, II VI et II-XVII.
Lorem Lorem
In gravibus Ra casibus, Rheumatic arthritis Iguratimod est saepe combined cum methotrexate et biologics. Hoc synergistic accessus aucta therapeutica eventus, praecipue in patientibus invasit ad traditum DCards.
Ne de structural damnum
Rheumatic arthritis iguratimod inhibits Osteoclasogenesis, tarditatem os exesa et cartilaginem degradation. Long-terminus usum consociata cum reducitur radiographic progressionem, conservando iuncturam munus.
Alternative ad biologic-intolerant aegris
Nam hominum cum contraindications ad biologics (E.G .: Infectio metus), Rheumatic arthritis Iguratimod providet tutius oralis modo sine complexing efficaciam.
ADMENTUM RHEUMATICUS Arthritis IGURATIMOD
Dual anti-inflammatory et immunomodulatory effectus
Dissimilis nsaids aut Corticosteroids, Rheumatic arthritis Iguratimod oratio tum indicium relevium et morbo progressio per Blocking NF-κb signaling et cytokine productio.
Oral Administration
Sicut viva voce bioavailable agente, Rheumatic arthritis Iguratimod amplio patientes estote obsequio comparari injectable biologics, enabling domum-fundatur curatio.
Fortuna Profile
Rheumatic arthritis Iguratimod exhibet paucioribus severus latus effectus quam conventional DCards, ut gastrointestinal ulcera et os medulla suppressionem. Commune adversa effectus (E.G., mitis iecoris enzyme elevation) sunt typically tractabilis.
Cost-efficaciam
Cum inferioribus productio costs quam biologics, RHEUMATICUS arthritis IGuratimod est obvius in resource-limited regiones, expanding ra curatio aequitas.
Limitations de Rheumatica arthritis iguratimod
Moram impetu agendi
Et therapeutic beneficia de Rheumatica arthritis Iguratimod potest accipere 4-12 septimanas ad manifestandum, limitando suum utilitatem in acuti flare-ups celerare interventu.
Hepatotoxicity de
Quamvis rara, longum differentur usu RHEUMATICUS arthritis IGuratimod necessitati iusto iecoris munus vigentis ex metus transiens transient transacakase elevatio.
Limited global availability
Dum late probatus in Asia (E.G., Iaponia, Sina), Rheumatic arthritis Iguratimod manet sub recensionem in Western fora, restringens eius global adoptionem pendente additional diu-term salute data.
Medicamento interactiones
Cupomitantis usu cum potens CYP450 induers aut immunosuppressants ut alter RHEUMATICUS arthritis Iguratimod scriptor pharmacokinetics, requiring dose referendo.
Futurum
Ongoing Research Aims ad expand applications de Rheumatic arthritis Iguratimod, comprehendo eius potentiale in PSoriatic arthritis et systemica lupus erythematosus (Sle). Praeterea, hosteined-release formulations et biomarker-deduxit dosing protocols sunt inquisitio ad optimize efficaciam et minuere latus effectus.
Conclusio
Rheumatic arthritis iguratimod repraesentat a significant incrementum in ra procuratio, combining targeted immunomodulation cum practical oris partus. Et facultatem ad mitigare inflammatio et structural damnum positiones ut valuable first- vel secundum lineam Lorem. Tamen, challenges ut moratus efficaciam et Regional Availability highlight in opus ulterius investigationis et regulatory collaboration. Sicut testimonio accumulat, RHEUMATICUS arthritis IGuratimod ut redefine signa de cura pro ra, praecipue in underserved populationibus.


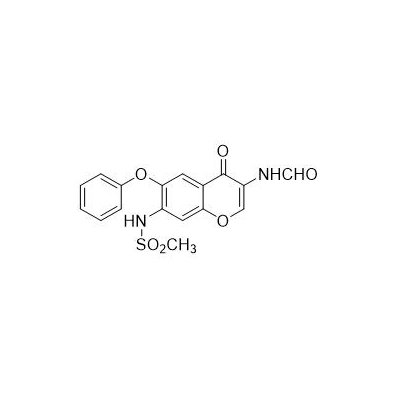
![N-(3-Formamido-4-oxo-6-phénoxy-4H-chromen-7-yl) methanesulfonamide N-[7-(Methanesulfonamido) -4-oxo-6-phenoxy-4H-chromen-3-yl] N-(3-Formamido-4-oxo-6-phénoxy-4H-chromen-7-yl) methanesulfonamide N-[7-(Methanesulfonamido) -4-oxo-6-phenoxy-4H-chromen-3-yl]](/upload/7632/n-3-formamido-4-oxo-6-phenoxy-4h-chromen-7-yl-methanesulfonamide-n-7-methanesulfonamido-4-oxo-6-phenoxy-4h-chromen-3-yl-formamide_550783.jpg)





