- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Sinae Active Pharmaceutical Ingredient (API) fabrica, supplementum, Factory
Jiangsu Run'an Pharmaceutical Co, Ltd., subsidiaria omnino possessa de Jiangsu Chiatai Qingjiang Pharmaceutical Co, Ltd., modernus summus tech pharmaceuticus societas specialis in R&D, productionis et venditio fertilitatis ordinandae medicinae et varietatis APIs. Project constructionem mense Novembri 2018 incepit, LIX mu aream obtegens, cum tota obsidione 160 decies Yuan et tota area aedificium 25000 quadratum metrorum.
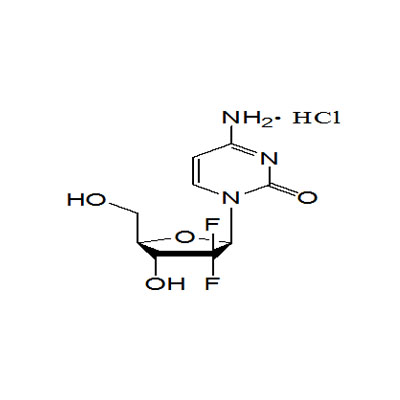
Praecipua activum medicamentum pharmaceuticum (API) producta: Hydrochloridem Gemcitabinum, Celecoxib, bromhexinum hydrochloridum, Iguratimod, Apremilast, Citrate Tofactinib, Crisaborole, Urapidil Hydrochloride, Sugammadex Natrium, Garlicin, Dexmedetomidine Hydrochloride, Rocuronium Bromidum, Finerenone, etc. elit mundi genus facti et productorum gignentium opificem per constantem emendationem et innovationem in technologiarum, et occasiones praeclarum negotium praebent sodalibus nostris per orbem terrarum. Munus nostrum ad praestantes operas emptores nobis permisit solidas relationes cum sociis nostris toto orbe includere.
- View as
Urapidil hydrochloride API
Urapidil hydrochloride API est ad medicamentum hypertensionis essentialis.
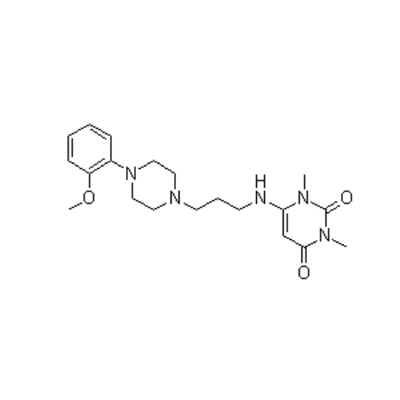
Lege plusMitte InquisitionemII-amino-3,5-Dibromo-n-cyclohexyl, n-methylbenzylamine hydrochloride
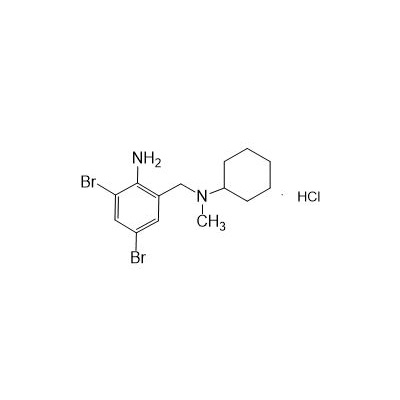
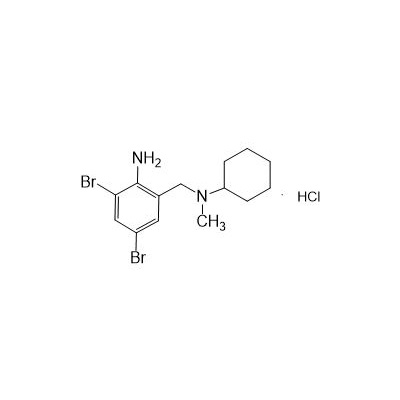
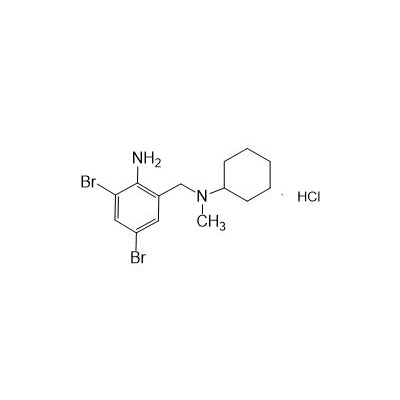
Seres Product Name: Bromhexine Hydrochloride
Seres aliases: Bromhexine hydrochloride; Hydrochloride bromhexylamine; Benzylcyclohexylamine bromide hydrochloride; II-amino-3,5-Dibromo-n-cyclohefyl, n-methylbenzylamine hydrochloride; N- (II-amino-3,5-Dibromobenzyl) -n-Methylyclohexylamine hydrochloride;
Latina Product Name: Bromhexine Hydrochloride
CAS # 611-75-6
N-(II-Amino-3,5-Dibromobenzyl) -n -N-Methylyclohexylamine Hydrochloride
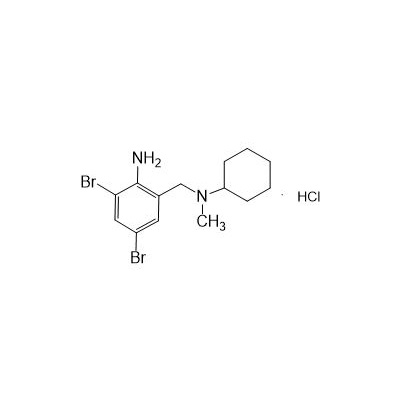
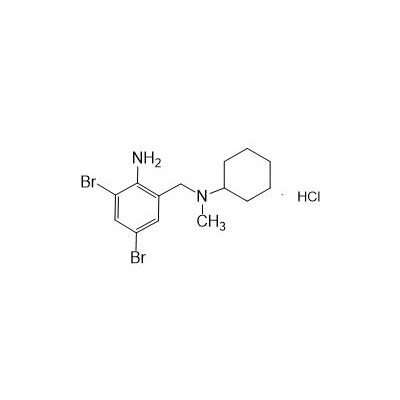
Seres Product Name: Bromhexine Hydrochloride
Seres aliases: Bromhexine hydrochloride; Hydrochloride bromhexylamine; Benzylcyclohexylamine bromide hydrochloride; II-amino-3,5-Dibromo-n-cyclohefyl, n-methylbenzylamine hydrochloride; N- (II-amino-3,5-Dibromobenzyl) -n-Methylyclohexylamine hydrochloride;
Latina Product Name: Bromhexine Hydrochloride
CAS # 611-75-6
CAS NO.611-75-6
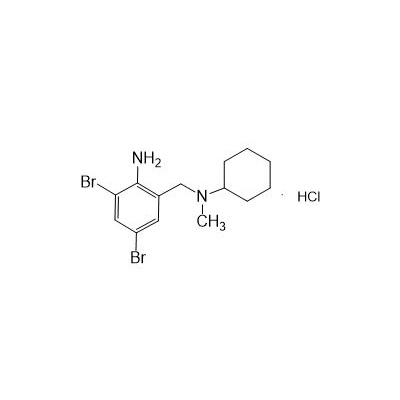
Seres productum nomen: Bromhexine hydrochloride
Seres aliases: hydrochloride bromhexine; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N hydrochloridum methylbenzylamine; N- (2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloridi;
Anglicus productum nomen: Bromhexine Hydrochloride
CAS NO.611-75-6
CAS 611-75-6
Seres productum nomen: Bromhexine hydrochloride
Seres aliases: hydrochloride bromhexine; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N hydrochloridum methylbenzylamine; N- (2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloridi;
Anglicus productum nomen: Bromhexine Hydrochloride
CAS 611-75-6
611-75-6
Seres productum nomen: Bromhexine hydrochloride
Seres aliases: hydrochloride bromhexine; bromhexylamine hydrochloride; benzylcyclohexylamine bromide hydrochloride; 2-amino-3,5-dibromo-N-cyclohexyl-N hydrochloridum methylbenzylamine; N- (2-Amino-3,5-dibromobenzyl)-N-methylcyclohexylamine hydrochloridi;
Anglicus productum nomen: Bromhexine Hydrochloride
Cas#611-75-6