- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
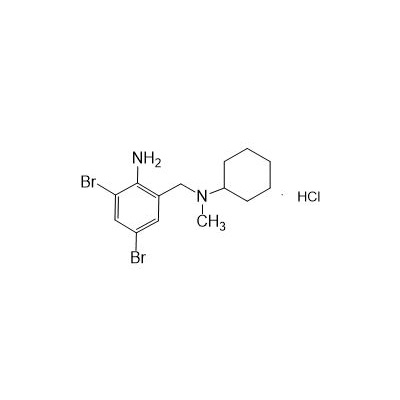
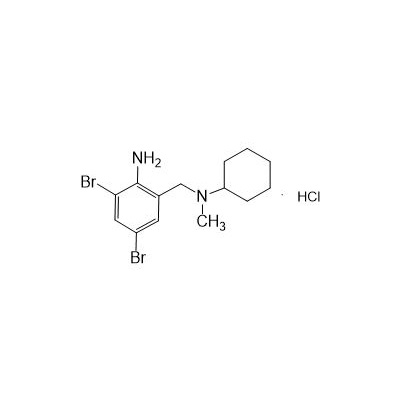
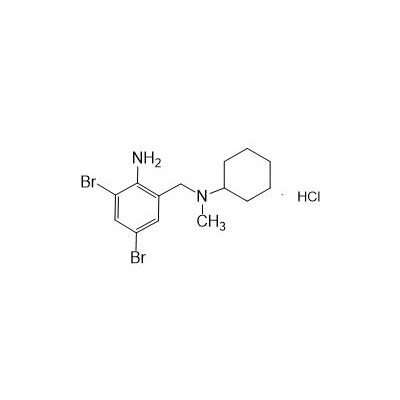
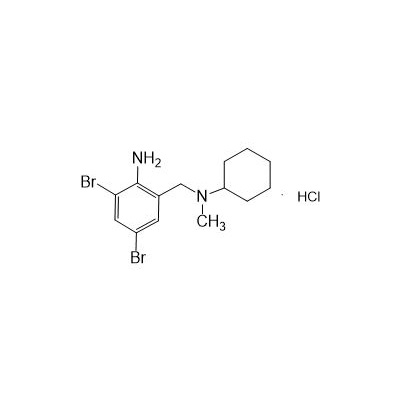
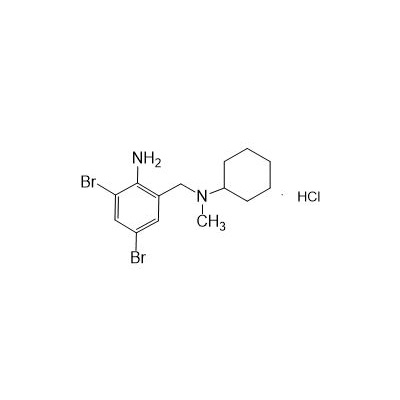
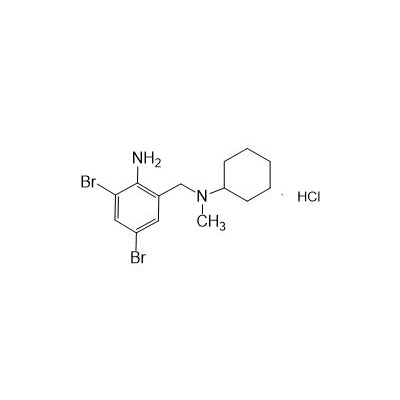
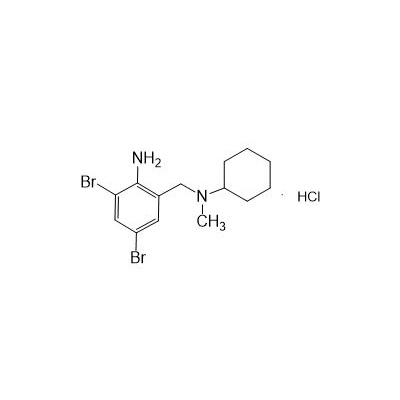
N-(II-Amino-3,5-Dibromobenzyl) -n -N-Methylyclohexylamine Hydrochloride
Seres Product Name: Bromhexine Hydrochloride
Seres aliases: Bromhexine hydrochloride; Hydrochloride bromhexylamine; Benzylcyclohexylamine bromide hydrochloride; II-amino-3,5-Dibromo-n-cyclohefyl, n-methylbenzylamine hydrochloride; N- (II-amino-3,5-Dibromobenzyl) -n-Methylyclohexylamine hydrochloride;
Latina Product Name: Bromhexine Hydrochloride
CAS # 611-75-6
Mitte Inquisitionem
Formula

Seres Product Name: Bromhexine Hydrochloride
Seres aliases: Bromhexine hydrochloride; Hydrochloride bromhexylamine; Benzylcyclohexylamine bromide hydrochloride; II-amino-3,5-Dibromo-n-cyclohefyl, n-methylbenzylamine hydrochloride; N- (II-amino-3,5-Dibromobenzyl) -n-Methylyclohexylamine hydrochloride;
Latina Product Name: Bromhexine Hydrochloride
CAS # 611-75-6
M. Formula: C14H21BR2CLN2
M. pondus: 412.6
Et proprietatibus specie: Alba solidae
Domesticis Registration Number API: Y20170001111
Usus: usus est acuti et longos BRONCHITIS, suspiriosis, bronchiectasis, et emphysema. Praesertim idoneos qui difficultatem tussis alba glutinosus sputum et discrimine emergentia per extensive impedimentum parva bronchi sputum.
N- (II-amino-3,5-dibromobenzyl) -n -en -en -en -nhylyclohexylamine hydrochloride est ad respiratorii medicamento et tussis cum pituitam medicamento.
N- (II-Amino-3,5-Dibromobenzyl) -n-Methylyclohexylamine hydrochloride, a multifaceted compositis in modern medicinales chemiae
Inventionis N-(II-amino-3,5-Dibromobenzyl) -n-Methylyclohexylamine hydrochloride marcas a significant milestone in progressionem novæ bioactive moleculis. Hoc structuram unique compositis, propria per suam brominated aromatic core et cyclohexlamine backbone, quae emersit sicut promissum candidatum in medicinales et diagnostic applications. Et synthesis, physicochochemical proprietatibus et pharmacological potentiale permanere captivate investigatores trans disciplinis.
I. synthesis et structural features
Praeparatio N-(II-amino-3,5-Dibromobenzyl) -n-Methylyclohexhexylamine hydrochloride involves multi-gradus processus incipiens a 3,5-II-nitrobenzaldehyde. Reductive amination cum methylcylochexylamine, sequitur a catalytic hydrogenation ad redigendum Nitro coetus ad a Amine, cedit libera base. Finalis curatio cum hydrochloric acidum producit hydrochloride sal, enhancing eius solubility et stabilitatem. X-Ray Crystallography reveals a detorta sella conformatio in Cyclohefyl circulum, dum bromine atomos ad positions III et V partum sternic et electronic effectus, quae influentiam receptor binding.
II. Pharmacological Applications
N-(II-Amino-3,5-Dibromobenzyl) -n -en -Nhylyclohexylamine Hydrochloride monstrat insignis affinitate Serotonin receptores (V, HT2A / 2C) in preclinical studia. Et IC50 valorem XII NM contra V, HT2A suggerit potentiale ut antipsychotic agente. Molecular docking simulationes ostendere quod bromine atomorum formam halogen vincula cum Thren and Ser159 residua, cum protonated Amine interacts cum Asp155-a key mechanism underlying eius selectivity. Current iudiciis explorare efficaciam in tractando schizophrenia et migraine perturbationes.
III. Partes in Diagnostic Imaging
Quam therapeutics, n- (II-amino-3,5-dibromobenzyl), -n, methylcylhexylamine hydrochloride servit quasi precursor ad radiolabeled probat. Isotopic substitutione bromine cum fluorine-XVIII datae pet imaging of neurotransmitter systems. In Murine exempla, in ^ 18f-intitulatum derivative exhibet celeri sanguine-cerebrum obice penetrationem et specifica cumulus in corticali regiones, highlighting eius utilitatem in Mapping V-HT2A distribution in vivo.
IV. Stabilitatem et formula provocationes
Quamvis eius promissionem, n- (II, amino-3,5-dibromobenzyl) -n -n-methylyclohexylamine hydrochloride munera formula cratibus debitum ad hydroscopicity et ps dependens degradation. Accelerated stabilitatem studiis (XL ° F / LXXV% RH) Ostende VIII% Decomposition super XII weeks, praesertim via dehydrohalogenation. Nanoencapsulation in plga Nanoparticles est melius bioavailability per CCC%, addressing eius limitata oris effusio.
V. Future Directions
Ongoing Research in N-(II-Amino-3,5-Dibromobenzyl) -n, Methylyclohexylamine Hydrochloride focuses super structural Optimization. Derivatives cum trifluatoromethyl coetus ad locum IV ostende enhanced metabolicae stabilitas in hepaticae microsome assays. Collaborative nisus inter Computational Chemists et pharmacologists intendunt ad statera eius receptor selectivity et pharmacokinetic profile, in potentia unlocking applications in tristitia et neurodegenerative morbo.