- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
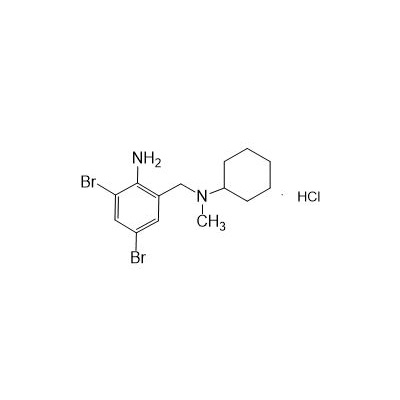
Bromhexine Hydrochloride API
Seres Product Name: Bromhexine Hydrochloride
Seres aliases: Bromhexine hydrochloride; Hydrochloride bromhexylamine; Benzylcyclohexylamine bromide hydrochloride; II-amino-3,5-Dibromo-n-cyclohefyl, n-methylbenzylamine hydrochloride; N- (II-amino-3,5-Dibromobenzyl) -n-Methylyclohexylamine hydrochloride;
Anglicus Product Name: Bromhexine Hydrochloride API
CAS # 611-75-6
Mitte Inquisitionem
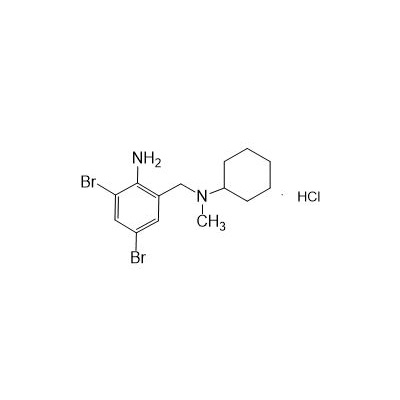
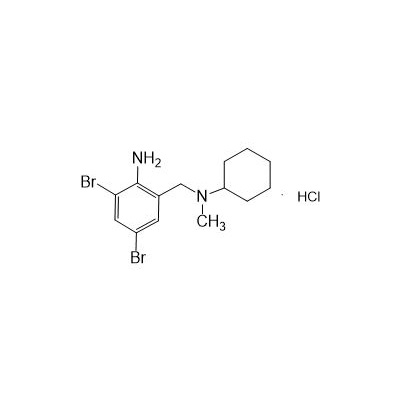
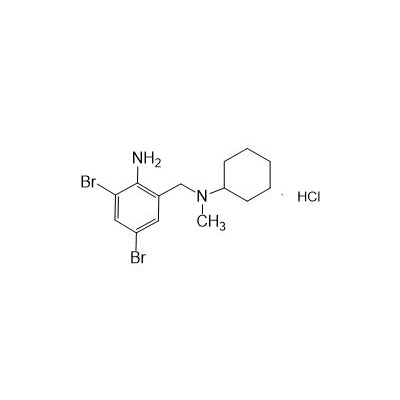
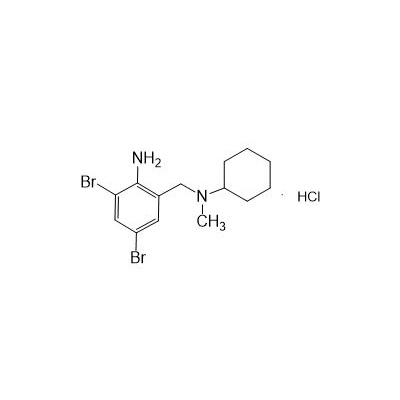
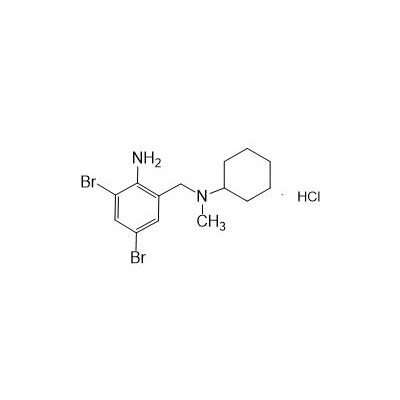
Formula

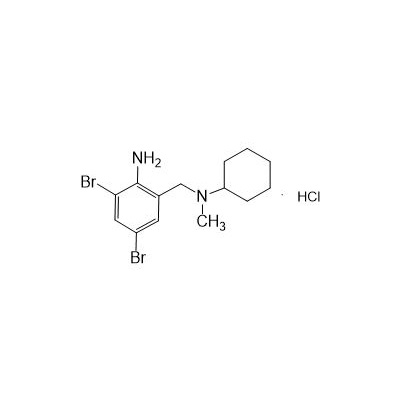
Seres Product Name: Bromhexine Hydrochloride
Seres aliases: Bromhexine hydrochloride; Hydrochloride bromhexylamine; Benzylcyclohexylamine bromide hydrochloride; II-amino-3,5-Dibromo-n-cyclohefyl, n-methylbenzylamine hydrochloride; N- (II-amino-3,5-Dibromobenzyl) -n-Methylyclohexylamine hydrochloride;
Anglicus Product Name: Bromhexine Hydrochloride API
CAS # 611-75-6
M. Formula: C14H21BR2CLN2
M. pondus: 412.6
Et proprietatibus specie: Alba solidae
Domesticis Registration Number API: Y20170001111
Usus: usus est acuti et longos BRONCHITIS, suspiriosis, bronchiectasis, et emphysema. Praesertim idoneos qui difficultatem tussis alba glutinosus sputum et discrimine emergentia per extensive impedimentum parva bronchi sputum.
Bromhexine Hydrochloride API est ad respiratorii medicamento et tussis cum pituitam pharmacum.
Bromhexine Hydrochloride API: A Aliquam Overview
Introductio
Bromhexine Hydrochloride API (Active PHARMACEUTICAL) est mucolytic agente late agnita ad efficaciam in administratione perturbationes propria nimia mucus productio. Sicut derivativum alkaloid Vasicine, Bromhexine Hydrochloride API operatur per reducendo mucus viscositas, facilitating expecto, et meliorem airway alvi. Hoc articulum explorat pharmacological proprietatibus, therapeutic applications, vestibulum processus, et foro dynamica de Bromichi Hydrochloride API, extollit eius significationem in modern medicine.
Pharmacological mechanism
Bromhexine Hydrochloride api exerit mucolytic actio per Depolymerizing mucopolysaccharide fibris in bronchiales secretiones. Eam incitat productio de solo fluidi in tractu respiratorii, enhancing hydrolytic operatio enzymes ut Hyaluronidase. Praeterea, Bromhexine Hydrochloride API promovet regenerationem alveolaris surfactant, improving pulmone obsequium. Eius bioavailability et celeri effusio faciet illud malle choice in formulationibus targeting longos BRONCHITIS, suspiriosis et alias pulmonis morbos.
Medicamentum
Clinocally, Bromhexine Hydrochloride API est incorporatus in syrups, tabulis et injectables ad inscriptio acuti et longos respiratoriorum conditionibus. Studies Highlight eius annuncia munus in ageretur Covi-XIX related mucus retention, underscoring eius versatility. API scriptor facultatem ad synergize cum antibiotics per enhancing eorum penetratio in pulmone textuum adhuc lata est therapeutica scope. Regulatory agencies worldwide et probatus Bromhexine Hydrochloride API quasi tutum et effective mucolytic agente, solidifying eius loco in global pharmacopeias.
Vestibulum et qualis imperium
Et synthesis de Bromhexine Hydrochloride API involves multi-gradus eget processus, incipiens a Vasicine extraction aut synthetica derivatization. Stricte adhaesione ad bonum vestibulum exercitia (GMP) ensures princeps puritatis (> XCIX%) et obsequio cum pharmacopopopopein signa (E.G .: USP, EP). Advanced analyticae techniques, inter HPLC et NMR, sunt usus ad convalidandum API est identitatem, potentiam et absentia impudicitiis. Prioritze sustineri exercitia ut minimize environmental ictum in Bromhexine hydrochloride API productio.
Market dynamics
In global demanda ad Bromhexine Hydrochloride API est repulsi ab ortu respiratoriorum morbus invaluisset et expanding generica foro. Asia-Pacific domatetur productio, cum India et Sinis accounting pro super LX% de copia. Patentes Expirations et Sumptus-Efficens vestibulum non intensified competition, instituens innovation in formula technologiae. Bromhexine Hydrochloride API est projected est testis a Cagr of 4.8% a MMXXIII ad MMXXXX, reflectendo suum permanens relevance in curis.
Salus et regulatory considerations
Bromhexine Hydrochloride API exhibet prosperam salutem profile, cum adversa effectus typically limited ad mitis gastrointestinal perturbationibus. Regulatory corpora, quos possidet FDA et EMA, mandate rigorous preclinical et orci testing ut efficacia et salus. Post-Venalicium Cervus Programs continuously Monitor-Verus-World Eventus, Fideles Confidentia in Bromhexine Hydrochloride API, secundum therapies.
Futurum prospectus
Ongoing Research explorat Novel Applications of Bromhexine Hydrochloride API, ut Nanoparticle-fundatur Delivery Systems ad targeted actio. Collaborative nisus inter Academia et industria aim ad optimize sua pharmacokinetics et expand indicia. Sicut personalized medicina lucra tractu, Bromhexine Hydrochloride API est parit ut manere lapidem angularem in respiratoriorum therapeutics.
Conclusio
Bromhexine Hydrochloride API exemplificat intersection of traditional pharmacology et modern innovation. Eius probatum mucolytic efficaciam, copusti fabrica compacta et evolving applications, sustentat sustinuit ictum in global salutem. Ut respiratorii oneribus escalate, bromhexine hydrochloride API erit permanere ludere a Pivotal Partes in improvidus patientes estote eventus terrarum.